| |
 · www.tappi.org
· www.tappi.org
· Subscribe
to Ahead of the Curve
· Newsletters
· Ahead
of the Curve archived issues
· Contact
the Editor

|
|
|
|
Can gribble nibble waste into biofuel?
According to the University of York, researchers and scientists—led by University of York professors Professor Simon McQueen-Mason and Professor Neil Bruce—have discovered a new enzyme that could prove an important step in the quest to turn waste, such as paper, scrap wood and straw, into liquid fuel.
As reported on the university's website, the research centers around the destructive power of the tiny, wood-boring marine isopods called "gribble," which historically attacked the timber hulls of seafarers' ships and continue to wreak damage on wooden piers and docks in coastal communities around the world.
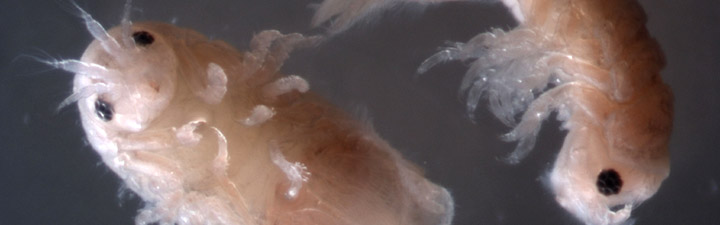 Photo by Simon Cragg, University of Portsmouth Photo by Simon Cragg, University of Portsmouth
Using advanced biochemical analysis and X-ray imaging techniques, scientists from the Centre for Novel Agricultural Products (CNAP) in the Department of Biology at York; the University of Portsmouth; and the US National Renewable Energy Laboratory in Golden, CO, have determined the structure and function of a key enzyme the gribble use to digest wood. This will help the researchers reproduce its effects on an industrial scale in a bid to create sustainable liquid biofuels. The research is published in the Proceedings of the National Academy of Sciences (PNAS), USA.
To create liquid fuel from woody biomass, such as wood and straw, the polysaccharides (sugar polymers) that make up the bulk of these materials must be broken down into simple sugars. These are then fermented to produce liquid biofuels. This process is difficult and expensive.
To find more effective ways of converting wood to liquid fuel, scientists are studying organisms that have evolved to live on a diet of woody matter, because these could provide a starting point for developing industrial enzymes to do the same.
Unusual Enzymes
As the University of York reports, gribble are voracious consumers of wood and have all the enzymes needed for its digestion. The enzymes attach to a long chain of complex sugars and then chop off small soluble molecules that can be easily digested or fermented. The researchers identified a cellulase (an enzyme that converts cellulose into glucose) from gribble that has some unusual properties and used the latest imaging technology to understand more about it.
Professor McQueen-Mason, of CNAP, says "Enzymes are proteins that serve as catalysts—in this case, one that degrades cellulose. Their function is determined by their three-dimensional shape. By studying the structure of the enzyme we have found a number of unusual features that could be of benefit to the biofuels industry."
While similar cellulase have been characterized from wood-degrading fungi, the enzyme from gribble shows some important differences. In particular, the gribble cellulase is extremely resistant to aggressive chemical environments and can work in conditions seven times saltier than sea water. Being robust to difficult environments means that the enzymes can last much longer when working under industrial conditions, so less enzyme will be needed. Understanding the structural basis for this robustness will help the researchers design more robust enzymes for industrial applications.
Professor McQueen-Mason explains, "This is the first functionally characterized animal enzyme of this type and provides us with a previously undiscovered picture of how they work.
"While this enzyme looks superficially similar to equivalent ones from fungi, closer inspection highlights structural differences that give it special features. For example, the enzyme has an extremely acidic surface and we believe that this is one of the features that contributes to its robustness."
The ultimate goal is to reproduce the effect of this enzyme on an industrial scale. Rather than trying to get the cellulase from gribble, the research team has transferred the genetic blueprint of this enzyme to an industrial microbe that can produce it in large quantities, in the same way that enzymes for biological washing detergents are made. By doing this they hope to cut the costs of turning woody materials into biofuels.
Adds Professor Bruce, "The robust nature of the enzymes makes it compatible for use in conjunction with sea water, which would lower the costs of processing. Lowering the cost of enzymes is seen as critical for making biofuels from woody materials cost effective. Its robustness would also give the enzymes a longer working life."
Bringing researchers together
This work is part of the Biotechnology and Biological Sciences Research Council (BBSRC) Sustainable Bioenergy Centre, a £24 million (US$31.6 million) investment that brings together six world-class research programs to develop the United Kingdom's bioenergy research capacity. Visits between the research teams were supported by a BBSRC-USA Partnering Award.
BBSRC Chief Executive Douglas Kell says, "This is an exciting step in realizing the potential of these important enzymes. If we can harness them effectively, waste materials could be used to make sustainable fuels. It's a double bonus: avoiding competition with land for food production, as well as utilizing unused materials from timber and agricultural industries."
For further information, see the original press release from the University of York. The Centre for Novel Agricultural Products (CNAP) is an award-winning strategic research center based in the Department of Biology at the University of York. CNAP is dedicated to realizing the potential of plants as renewable, low-cost factories that produce high-value chemicals and biofuels. Lab-based discoveries are translated into practice in partnership with industry.
The BBSRC invests in world-class bioscience research and training on behalf of the UK public. Its aim is to further scientific knowledge; to promote economic growth, wealth, and job creation; and to improve quality of life in the UK and beyond.
The paper "Structural characterization of the first marine animal Family 7 cellobiohydrolase suggests a mechanism of cellulase salt tolerance" is published in PNAS Early Edition; read the abstract here.
For a modest investment of $174, receive more than US$ 1000 in benefits in return.
Visit www.tappi.org/join for more details. |
|




